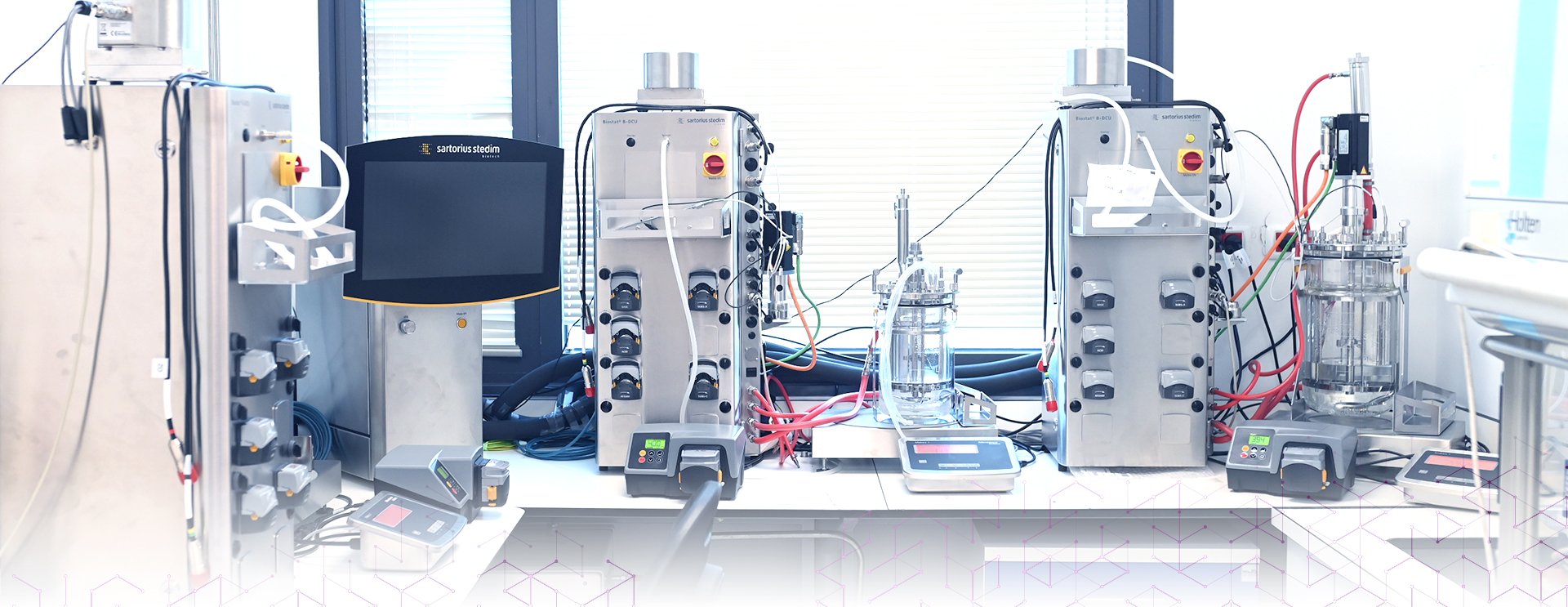
Process development and scale-up
Scinai offers cost-effective, flexible and scalable, comprehensive process development & scale-up solutions, based on our vast experience in developing and executing pre-clinical and clinical plans for recombinant proteins. Our expert team guides you through the drug research and development process, reducing risks and supporting you every step of the way.

Services
Downstream purification
From lab-scale development to optimized downstream production process
Upstream Fermentation
Fermentation process development for bacteria yeast and fungi. Lab-scale process development and optimization.
Clone selection
Protein bio-processing- getting to the right clone for the highest yield and quality as early as possible
Process optimization & scale-up
Process development and optimization under design of experiments (DOE) methodology incorporating Quality by Design principles.
Deep In-house Process Development and Scale-up Experience
Zohar Gadri, MSc
-
Technical R&D Upstream Process Team Leader
Processes development and optimization; Protein purification from laboratory to GMP conditions
Barry Cohen, MSc
-
Technical R&D Downstream Process Team Leader
Processes development and protein purification from laboratory to GMP standard conditions.

REQUEST MORE INFO
Get in touch to learn more about our comprehensive CDMO solutions and request a quote for your project






